

#Accu chek inform ii strips free
If this is not possible then we will happily dispatch your order free – whatever its size! Please note that some larger items of equipment may still incur a delivery charge especially if you require delivery to anything other than the ground floor – we will advise you what these charges may be as soon as we receive your order. This record will be updated as the status changes.Delivery is FREE on any order size or amount however to maintain our high level of service and to cover our costs, we do ask that you try and make your order above £75 excluding VAT. Learn more about medical device recalls.Ģ Per FDA policy, recall cause determinations are subject to modification up to the point of termination of the recall.ģ The manufacturer has initiated the recall and not all products have been corrected or removed. The record is updated if the FDA identifies a violation and classifies the action as a recall, and it is updated for a final time when the recall is terminated. Worldwide distribution - US Nationwide distribution.ġ A record in this database is created when a firm initiates a correction or removal action. Urgent Medical Device Correction(UMDC) by phone at 1-80 or on our website at under Contact Us for email or chat. Dispose of the affected test strips and vial according to your local guidelines.Ĭontact Accu-Chek Customer Care if you have questions regarding the information in this Complete and return the business reply letter to the recalling firm.ĥ. On our website at under Contact Us for email or chat. Contact Accu-Chek Customer Care for product replacement if you open a sealed carton and any of the vials inside meet the criteria listed above by phone at 1-80 or DO NOT perform control testing if you open a sealed carton and ay of the vials inside meet the criteria listed above.ģ. Anything prevents the cap from closing properlyĢ. You see any damage to the cap or vial, or The vial is open or damaged before using the test strips for the first time Check vials of affected test strips before use.
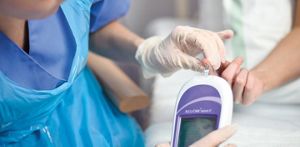
Inappropriate therapy decisions based on inaccurate results could lead to adverse health consequences.ġ. Customers were warned that open vials may expose the test strips to humidity, which might damage the strips and result in inaccurate results (such as positively biased or falsely too high results).

On July 28, 2021, the firm issued an Urgent Medical Device Correction to affected customers regarding the potential for open vials. An open vial might expose the test strips to humidity, which might damage the strips and result in inaccurate results, potentially leading to inappropriate therapy decisions and adverse health consequences. Test strip vials may open while inside sealed cartons during shipment. Since this is a labeling correction, this recall is not lot specific and is
#Accu chek inform ii strips code
System, test, blood glucose, over the counter - Product Code NBWĪccu-Chek Inform II Test Strip, Whole Blood Glucose Test System, Model number 05942891001 Class 2 Device Recall AccuChek Inform II Test Strip


 0 kommentar(er)
0 kommentar(er)
